EU Repatriation of APIs and Intermediates – Midas contributes to supply chain resiliency
Midas Pharma is actively working on solutions for shortages of pharmaceutical raw materials and disrupted supply chains. By using the Midas’ global network, we identify and establish alternative sources for products with high risk of failure, for example due to dependency on specific territories / limited number of suppliers (see also "Raw material shortages (April 2020)" ). We build on several years’ experience with this strategy, triggered by structural shortages in the industry.
“Over recent years, structural shortages of medicines have been building up, as a consequence of weaknesses in the supply chain. The recent COVID pandemic only exacerbated this existing problem, highlighting the urgent need to significantly improve the robustness of the supply chain and allowing the identification of vulnerabilities."
European Fine Chemicals Group (EFCG); Sector group of the European Chemical Industry Council (CEFIC); July 2020
-
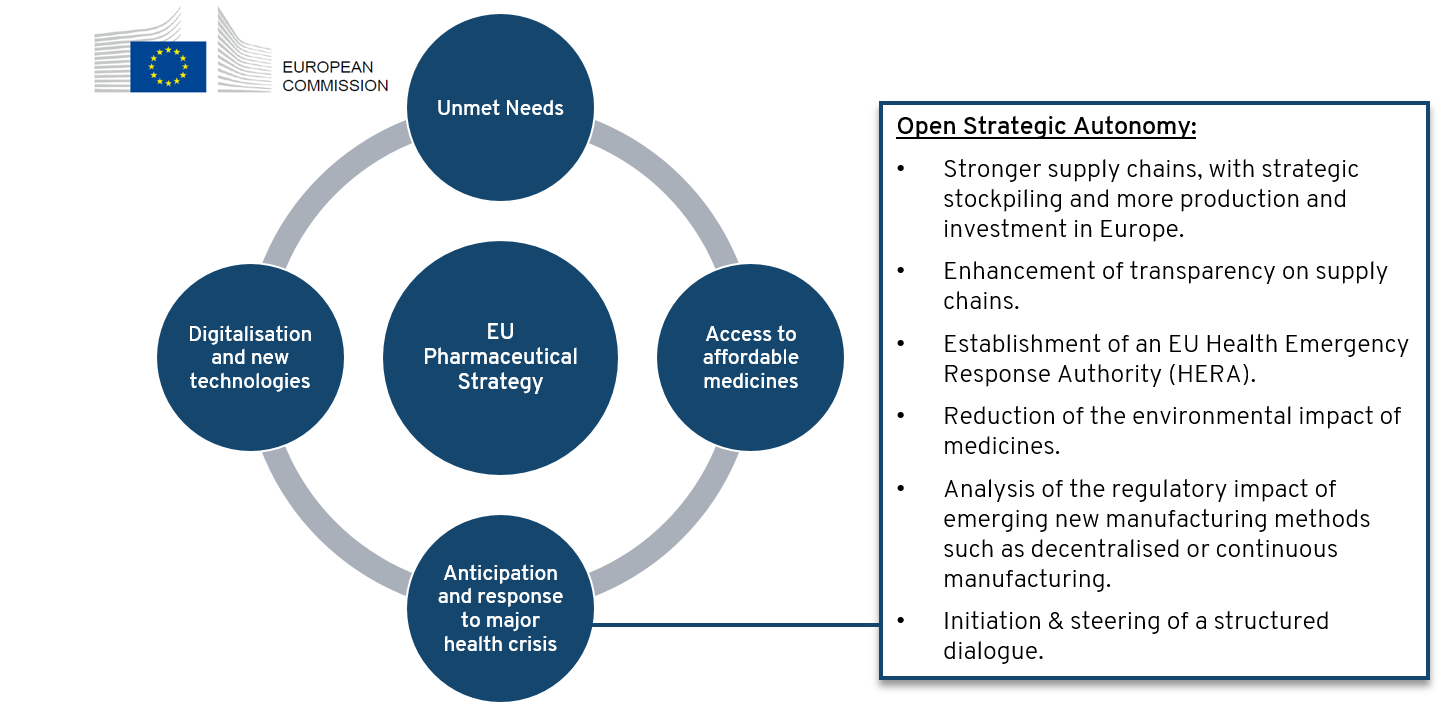
Security of Supply of essential medicines is one of the four pillars of the European Commission’s Pharmaceutical Strategy
On November 25th, 2020 European Commission released its Pharmaceutical Strategy for Europe with a framework to “ensure that patients have access to high-quality, effective and safe medicines”. The strategy consists of four pillars:
- Address unmet medical needs, by promoting promoting Research & development for innovative treatments (e.g. against antimicrobial resistance (AMR), rare diseases, cancer)
- Ensuring access to affordable medicines and promoting competitiveness & sustainability of the European pharmaceutical sector
- Enabling digital transformation and investment in innovation and new technologies
- One key element is to secure access to essential medicines also in times of major health crisis, by investing in Europe, building up robust supply chains and enhancing transparency. Further elements of building an EU strategic autonomy are illustrated in following scheme. As initial step, a structured dialogue was launched by the Commission on Feb. 26th, 2021 with the aim to set concrete measures to address weaknesses in the supply chain that could lead to EU drug shortages. Results are planned to be released by end of 2021. Proposals for relocation of production back to Europe are discussed in a recently released study commissioned by the Committee on International Trade (INTA) of the European Parliament.
Midas Pharma is contributing to the European Pharmaceutical Strategy in terms of strengthening (domestic) access to essential medicines. Shortages can occur on different levels of the pharmaceutical value chain and require individual solutions. Midas addresses this challenge through formation of a cross-functional team that gathers information on current and potential shortages on precursor, API or finished dosage form level. These information are applied to conduct risk assessments and to generate solutions with the help of Midas’ global network.
Contributions by Midas Pharma to supply chain resiliency and access to essential medicines in Europe:
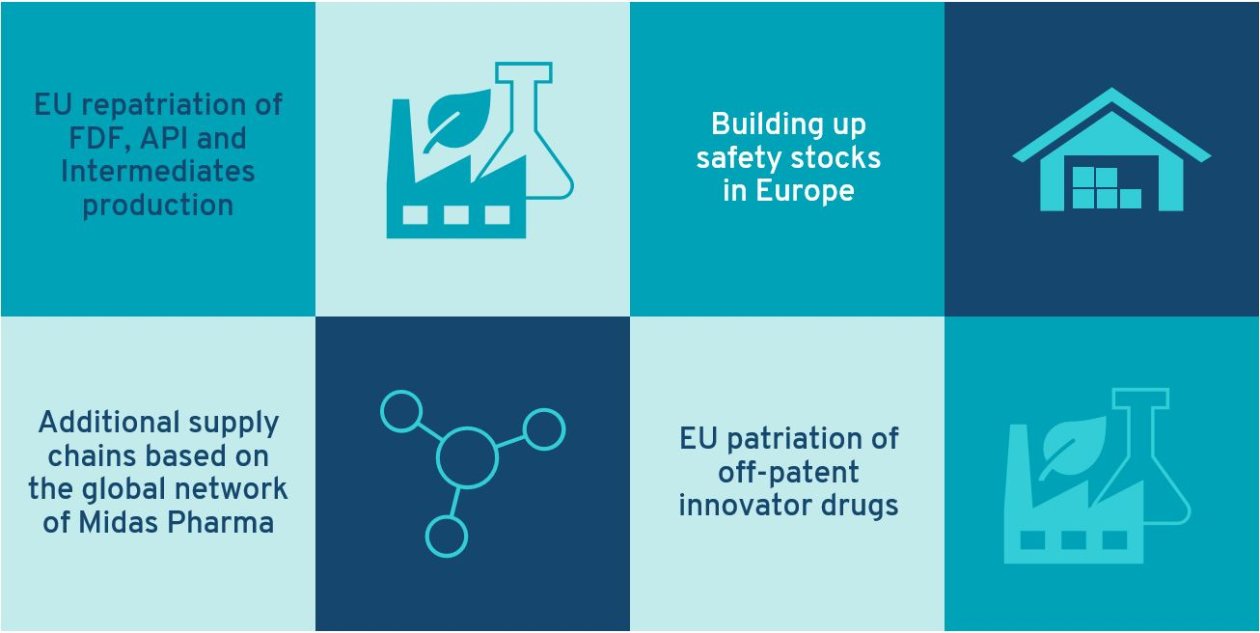
A) EU repatriation of FDF, API and Intermediates production
Transfer of technologies to Europe and reactivation of local production: The production of many active ingredients requires up to 10 synthesis steps, with the starting materials and intermediates often coming from only a few non-European sources. It is therefore important not only to focus on the manufacturing and production sites of active ingredients, but to analyze the entire synthesis pathway in order to better identify the reasons for shortages and to be able to take countermeasures. Midas Pharma has the experience and expertise to transfer the production of pharmaceutical precursors, active ingredients and finished products (FDF) to Europe or to reactivate local capacities. Specialized teams of chemists, pharmacists and regulatory experts are available for this purpose.
Midas Pharma is experienced and active in developing sustainable synthesis routes together with European development partners. For example, a more efficient enzyme-catalyzed synthesis has been developed and established, and the intermediate has been marketed already for several years. Similarly, Midas Pharma has initiated the development of an innovative continuous manufacturing process in Europe for an important molecule at significant financial expenditures.
B) Building up safety stocks in Europe
As a consequence of COVID-19 pandemic the call for building up safety stocks becomes stronger as opposed to the "just in time" model that has been popular for years. Due to its drug regulatory approvals and its worldwide network, Midas Pharma is able to assist in building up stockpiles in Germany/Europe, or to expand existing storage capacities.
C) Additional supply chains based on the global network of Midas Pharma
Midas Pharma is experienced in identifying current weaknesses in the supply/production chains of pharmaceutical products and is able to recommend or build alternative supply channels.
D) EU patriation of off-patent innovator drugs
As soon as products become off-patent, the manufacture is often subject to off-shoring, due to changing priorities at the innovator companies. Midas Pharma has developed a concept to counteract this process and to continue domestic production at European contract manufacturers.
If you are also interested in strengthening existing supply chains, present EU-local production capacities or are looking for alternative supply sources of pharmaceutical products / precursors, then we should exchange ideas!
Please do not hesitate to contact us!
Your Contact

Ben Schalke
Corporate Development
Senior Director International Partnering & Sourcing
Rheinstr. 49
55218 Ingelheim
Germany